
Keynote 189 plus#
METHODS Patients were randomly assigned (2:1) to receive pemetrexed and platinum plus pembrolizumab (n 410) or placebo (n 206) every 3 weeks for 4 cycles, then pemetrexed maintenance plus pembrolizumab or placebo for up to a total of 35 cycles. Together with the results from KEYNOTE-024, 12,13 the data from KEYNOTE-189 suggest that introducing immunotherapy as a first-line therapy may have a favorable long-term effect on outcomes.
Keynote 189 update#
Update to the latest version by going to Settings > General > Software Update. Objective: To characterize the benefit-risk profile of pemetrexed and platinum in combination with pembrolizumab in patients with non-squamous non-small cell lung cancer in the KEYNOTE-189 study, with reference to historical pemetrexed maintenance data from the PARAMOUNT, PRONOUNCE, and JVBL randomized studies. To access and use all the features of Apple Card, you must add Apple Card to Wallet on an iPhone or iPad with the latest version of iOS or iPadOS. PURPOSE: In KEYNOTE-189, first-line pembrolizumab plus pemetrexed-platinum significantly improved overall survival (OS) and progression-free survival (PFS) compared with placebo plus pemetrexed-platinum in patients with metastatic nonsquamous nonsmall-cell lung cancer (NSCLC), irrespective of tumor programmed death-ligand 1 (PD-L1) expression. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell.To get the newest features, make sure your devices are running the latest software version. KEYNOTE-189 demonstrated first-line pembrolizumab plus pemetrexed-platinum improves progressionfree survival (PFS) and overall survival (OS) in metastatic.

Apple Fitness+ requires iOS 14.3 or later, iPadOS 14.3 or later, watchOS 7.2 or later, and tvOS 14.3 or later.
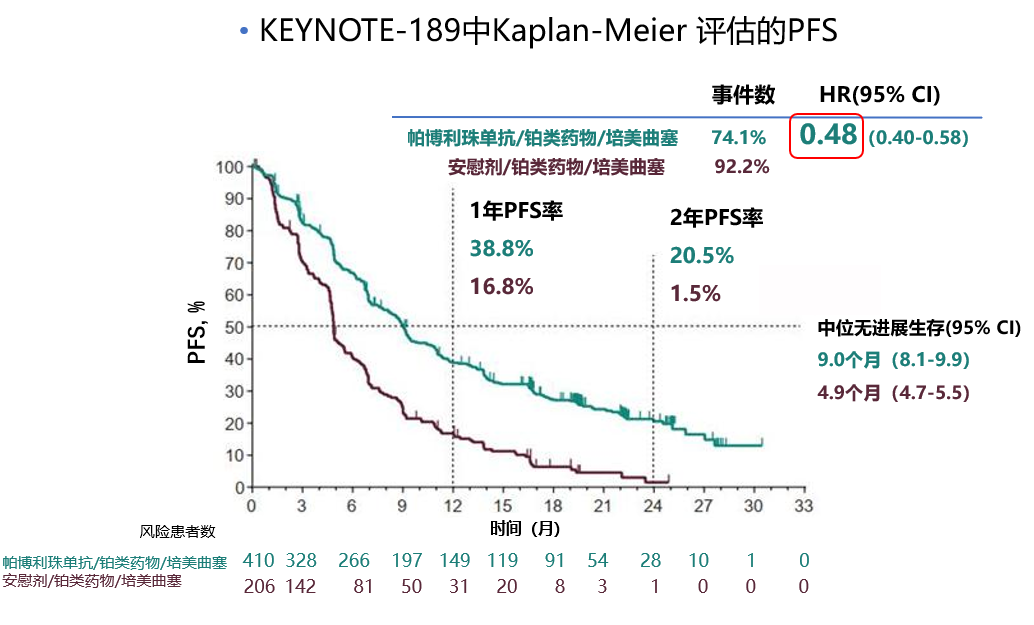
Keynote 189 trial#
The trial protocol and all amendments were approved by the appropriate ethics panel at each study center. In 1995 Aung San Suu Kyi delivered the keynote address at the Fourth. KEYNOTE-189 (NCT02578680) study design, eligibility criteria, as well as patient baseline and demographic characteristics, have been previously reported 12 and are briefly summarized in the Supplementary Materials.
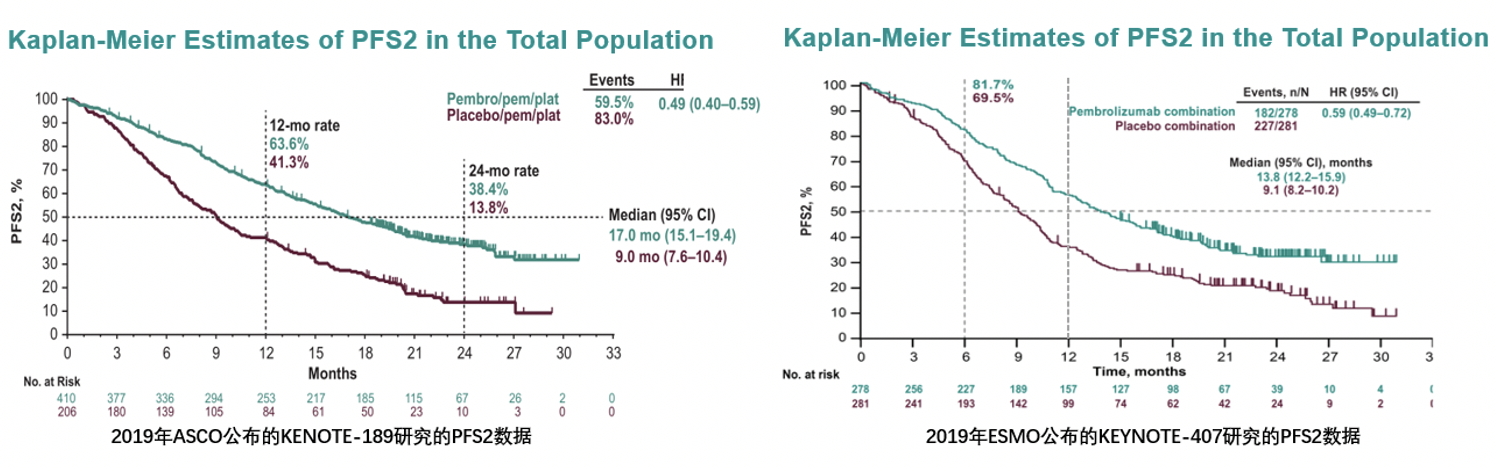


 0 kommentar(er)
0 kommentar(er)
